Our Mission
Multiple myeloma is a rare hematological malignancy affecting less than 5 per 10.000 people in the European Union. In clinical practice, there are a range of novel and chemotherapeutic treatment options available for patients. However, despite recent improvements in overall survival, myeloma remains a largely incurable disease. Our mission was to trial the emerging CAR-T cell technology in a cohort of European myeloma patients to understand whether we can overcome existing treatment limitations and find a more curative approach.
A clinical proof-of-concept for CAR-T cells has already been obtained in other hematological malignancies, e.g. B cell leukemia and lymphoma, resulting in dramatic and durable anti-tumour responses in patients. CARAMBA was the first clinical trial to target the myeloma-specific protein SLAMF7. The project had a mission to better understand the role of CAR-T therapy in myeloma and to explore regulatory and sustainable funding approaches for health care systems to ensure patients can access this type of innovation.
Our Approach
Cutting-edge CAR technology – made in the EU
Chimeric antigen receptors (CARs) are synthetic designer molecules that are capable of redirecting the specificity of T cells to potently eliminate tumour cells. We have developed a novel and unique CAR approach targeting the SLAMF7 antigen in myeloma and obtained proof-of-efficacy in comprehensive preclinical testing.
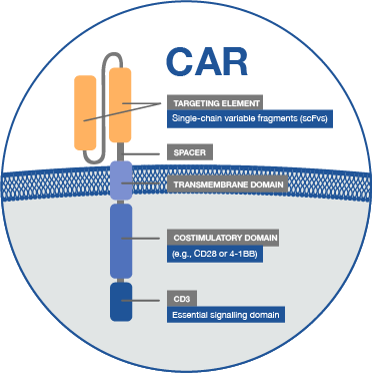
The novel SLAMF7 CAR design allows to select, detect, expand and delete CAR-T cells. Its proprietary SLAMF7-targeting domain is humanized to prevent immunogenicity and premature rejection of CAR-T cells. It is of particular importance, that SLAMF7 CARs are equipped with an EGFRt safety switch that can be triggered with anti-EGFR antibodies to deplete CAR-T cells in case of toxicity or after a therapeutic window once tumour clearance has been obtained.
A major achievement in the CAR-T design has been accomplished through our expertise in “Sleeping Beauty” transposition (i.e. the delivery of the CAR engineered gene into the human cells). In CAR-T this is typically done using viral systems or “vectors” which are efficient but may pose risks to the patient. Our approach guarantees a viral-free, high-level CAR gene transfer into safe genomic loci and is a major milestone for the development of a myeloma CAR therapy.
The manufacturing process for CAR-T cells using our novel Sleeping Beauty-based gene-transfer approach has been established under good manufacturing practice (GMP) conditions ensuring a rapid and high-quality production process.
SLAMF7 CAR-T clinical trial – 4 sites 30 patients
The clinical trial within CARAMBA is designed as a Phase I/II trial. Phase I is a dose escalation study and will explore the effective dose of the CAR-T cell product. For the Phase II part of the trial, 25 patients will be treated with the maximum tolerated dose of SLAMF7 CAR-T. After CAR-T cell infusion, all patients will be observed as inpatients (in hospital) for at least one week and then periodically as outpatients (at home).
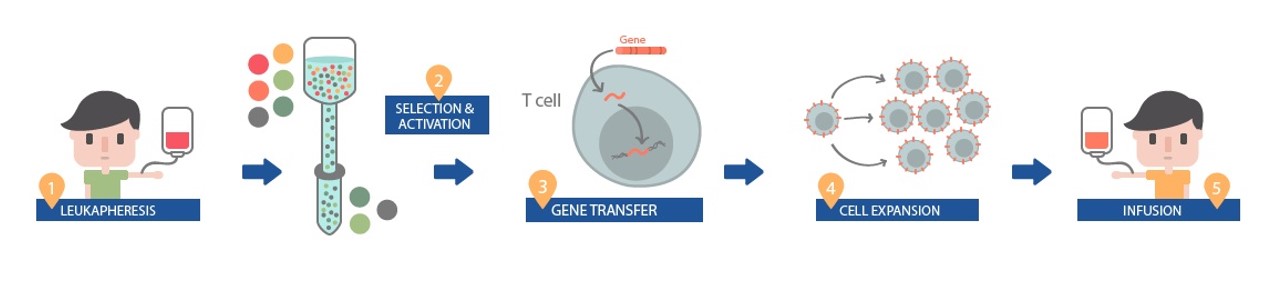
For the treatment the process is as follows:
- A patient’s white blood cells are extracted by leukapheresis
- The white blood cells are then separated to identify appropriate T-Cells (immune cells) to undergo adaptation for CAR-T
- “CAR” gene sequences are inserted into the DNA of the T cells to create the “CAR-T cells”. This engineers them to be able to find and target the SLAMF7 protein on myeloma cells.
- The modified T cells are then expanded ex-vivo (outside the body)
- Subsequently, T cells are infused back into the patients, where they can multiply when they encounter the targeted proteins and kill the targeted cancer cell.
-
Universitaetsklinikum Wuerzburg (Wuerzburg, Germany)
-
Universidad de Navarra (Pamplona, Spain)
-
Ospedale San Raffaele (Milano, Italy)
-
Centre Hospitalier Regional et Universitaire de Lille (Lille, France)
Our Impact
CARAMBA aimed to deliver a new therapeutic option for patients with myeloma and shape the next generation of medicine in rare diseases. CAR-T cell therapy is a novel, radically innovative therapeutic strategy with the attraction that a single infusion of CAR-T cells potentially offers curative anti-tumour efficacy and protection from relapse given their capacity to form long-lasting memory cells. From clinical trials with CD19 CAR-T cells in leukemia and lymphoma in the US, there is growing evidence of the impressive efficacy of CAR-T cell immunotherapy, even in patients where conventional treatments have failed or are no longer effective. The experience with CD19 CARs has shown that the onset of the therapeutic effect is rapid, durable, and begins immediately after infusion of the CAR-T cell product. Thus, cancer patients and physicians alike are eagerly awaiting the clinical introduction of this therapeutic modality in the EU, especially in rare cancers. We anticipate that where anti-myeloma efficacy of the SLAMF7-CAR is demonstrated in our trial, the wider work of the CARAMBA project will assist further exploration and its rapid implementation into clinical practice. Knowing that SLAMF7 is also expressed in other rare hematologic diseases, once proof-of-concept has been obtained, SLAMF7 CAR-T cells could also be used for these indications. In addition, CARAMBA has identified additional target antigens, that would allow to gear not only additional tumour indications but also other conditions, including autoimmune diseases, transplant rejection and degenerative diseases, ultimately aiming at establishing immunotherapy with engineered CAR-T cells as a broadly applicable therapeutic concept for rare diseases.
Our Timelines
CARAMBA is a EU-funded project that was started on 1st January 2018 with a project period of 66 months. Within its first 2 years, the GMP manufacturing process of the CAR-T product as well as the clinical trial approvals have been finalized. The first phase I patients have been recruited in 2020 and the study remains open after the project end. CARAMBA is highly committed to an open data policy and will present its generated data after completion of phase I and II at national and international meetings to physicians and the patients’ community.


