About Multiple Myeloma
Multiple myeloma is a rare hematological (blood) cancer affecting less than 5 per 10.000 people in the European Union. It is currently an incurable but treatable cancer and in European clinical practice, there are a range of novel and chemotherapeutic treatment options available for patients. If you are a patient or caregiver and require information on multiple myeloma and its treatment, please contact Myeloma Patients Europe, a large network of myeloma patient organisations. They can provide you with information and details on national support services where you live. You can contact them by emailing.
Please also visit their website to find out answers to the questions such as:
Myeloma Patients Europe develops information material about myeloma in general, and for CARAMBA in particular. On their website, you may find:
An explanatory video “How does CAR-T cell therapy work?“
CARAMBA Myeloma Pipeline Information in 5 languages (English, French, German, Italian and Spanish)
The CARAMBA STUDY
Engineering immune cells to treat Multiple Myeloma
The CARAMBA project approach is based on an innovative immunotherapy, known as Chimeric Antigen Receptor T-cell therapy (CAR-T). In the clinical trial, a type of white blood cell, which makes up part of the immune system (T-cells), will be collected from patients and will be equipped with a chimeric antigen-receptor (CAR). The CAR-T cells designed in CARAMBA are specifically looking for a protein called SLAMF7 which is expressed on the surface of myeloma cells. When reintroduced into patients’ bodies, they act like a sensor boosting the ability of the T-cells to find and destroy myeloma cells. In cancer medicine, the treatment of Non-Hodgkin-Lymphoma and of Acute Lymphocytic Leukemia with CAR T-cells has already been approved in US. With this EU-funded project, the innovative therapy is now also available for all European citiziens to treat Multiple Myeloma.
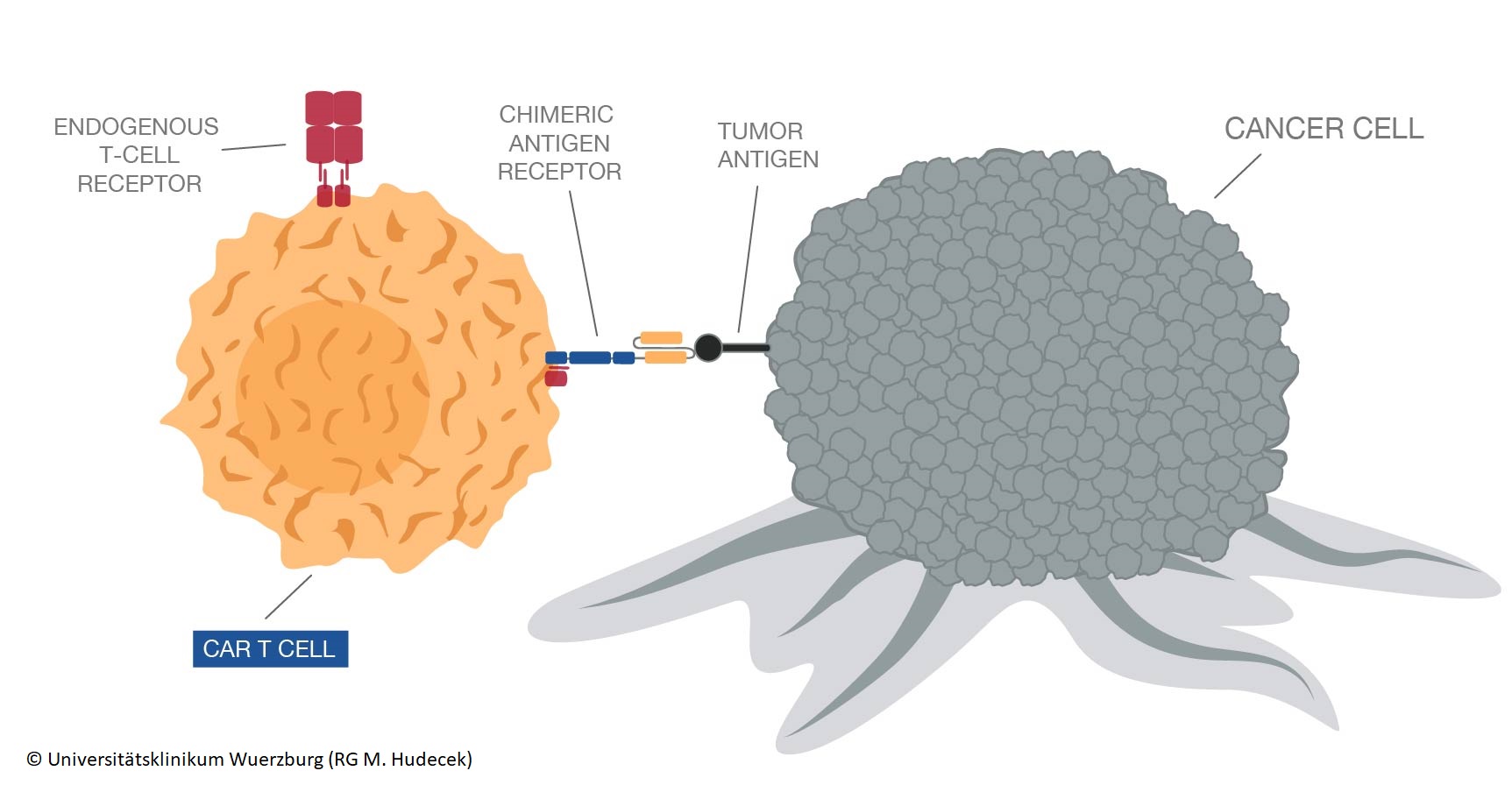
SLAMF7 CAR-T clinical trial – 4 sites 30 patients
The clinical trial within CARAMBA is designed as a Phase I/II trial. Phase I is a dose escalation study and will explore the effective dose of the CAR-T cell product. For the Phase II part of the trial, 25 patients will be treated with the maximum tolerated dose of SLAMF7 CAR-T. After CAR-T cell infusion, all patients will be observed as inpatients (in hospital) for at least one week and then periodically as outpatients (at home).
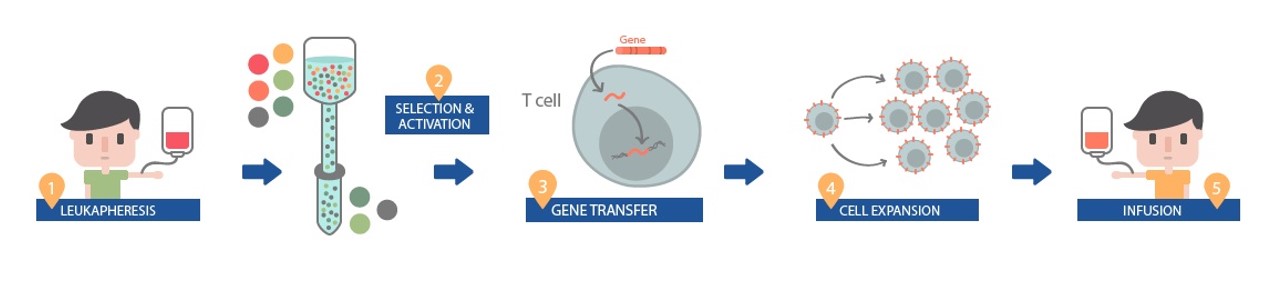
For the treatment the process is as follows:
- A patient’s white blood cells are extracted by leukapheresis
- The white blood cells are then separated to identify appropriate T-Cells (immune cells) to undergo adaptation for CAR-T
- “CAR” gene sequences are inserted into the DNA of the T cells to create the “CAR-T cells”. This engineers them to be able to find and target the SLAMF7 protein on myeloma cells.
- The modified T cells are then expanded ex-vivo (outside the body)
- Subsequently, T cells are infused back into the patients, where they can multiply when they encounter the targeted proteins and kill the targeted cancer cell.
The safety and efficacy of SLAMF7 specific CAR-T cells is assessed in multiple myeloma patients through a small Phase I/II clinical trial involving around 30 patients. CARAMBA started its screening for eligible myeloma patients for Phase I in 2020, and recruited the first patient in July 2020. The CARAMBA trial is open to all EU citizens and each of the 4 clinical sites is screening for patients:
- Universitaetsklinikum Wuerzburg (Wuerzburg, Germany)
- Universidad de Navarra (Pamplona, Spain)
- Ospedale San Raffaele (Milano, Italy)
- Centre Hospitalier Regional et Universitaire de Lille (Lille, France)
Contacts For Patients
Germany
For CARAMBA
Universitaetsklinikum Wuerzburg
TBD after initiation of the trial
Email
TBD
For general questions on myeloma
AMM-Online
Johan Creemers
Email
www.myelom.org
Spain
For CARAMBA
Universidad de Navarra
Dra. Paula Rodriguez-Otero
Email
00 34 948 296397
For general questions on myeloma
Comunidad Española de Pacientes con Mieloma Múltiple
Teresa Regueiro
Email
www.comunidadmielomamultiple.com
France
For CARAMBA
Centre Hospitalier Regional et Universitaire de Lille
Alexandra Preau
Email
For general questions on myeloma
Association Française des Malades du Myélome Multiple
Bernadette Isoir
Email
www.af3m.org
Germany
TBD after initiation of the trial
TBD
AMM-Online
Johan Creemers
Email
www.myelom.org
Spain
Universidad de Navarra
Dra. Paula Rodriguez-Otero
Email
00 34 948 296397
Comunidad Española de Pacientes con Mieloma Múltiple
Teresa Regueiro
Email
www.comunidadmielomamultiple.com
Italy
Ospedale San Raffaele
Grazia Consoli
Email
France
Centre Hospitalier Regional et Universitaire de Lille
TBD
Email
TBD
Association Française des Malades du Myélome Multiple
Bernadette Isoir
Email
www.af3m.org


